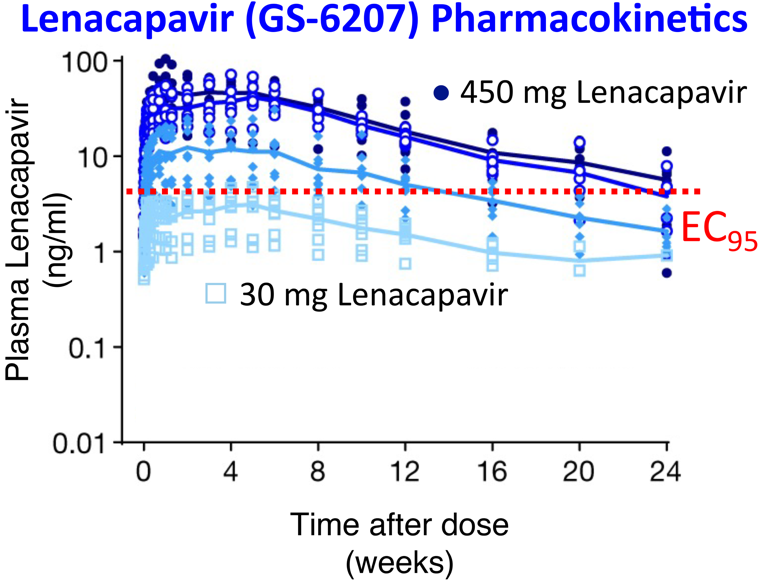
Antiretroviral drugs have saved the lives of millions living with HIV/AIDS. However, problems with drug resistance limit available treatments, and inconsistent adherence to daily dosing schedules can lead to poor outcomes and new infections. Long-acting drugs that can overcome drug resistance by targeting new classes of viral proteins are therefore needed. University of Utah Health researcher Wesley Sundquist, PhD, and colleagues performed mechanistic studies and completed a phase 1 clinical trial of Lenacapavir, a molecule that inhibits the HIV capsid (outer shell). Lenacapavir is long-acting due to its high potency, slow release from the injection site, and slow clearance from the body. Single doses of Lenacapavir injected under the skin maintained antiviral concentrations for more than 6 months. The study validates therapies that target the HIV capsid, and demonstrates the potential of Lenacapavir as a long-acting agent to treat and prevent HIV infections. Lenacapavir was developed by Gilead Sciences, building on studies of HIV capsid structure and function from the Sundquist and Chris Hill, DPhil, laboratories (and others). Following a successful phase 3 trial, Lenacapavir has now been approved for use in Europe, and use in the US is pending FDA approval.
References:

Clinical targeting of HIV capsid protein with a long-acting small molecule. Link JO, (Sundquist WI), et al. Nature. (2020) Aug;584(7822):614-618.

